F. Diegelmann, V. Tritschler, S. Hickel, N.A. Adams (2016)
Combustion and Flame 163:414-426. doi: 10.1016/j.combustflame.2015.10.016
We analyse results of numerical simulations of reactive shock-bubble interaction with detailed chemistry. The interaction of the Richtmyer–Meshkov instability and shock-induced ignition of a stoichiometric H2-O2 gas mixture is investigated. Different types of ignition (deflagration and detonation) are observed at the same shock Mach number of Ma = 2.30 upon varying initial pressure.
Due to the convex shape of the bubble, shock focusing leads to a spot with high pressure and temperature. Initial pressures between p0 = 0.25 − 0.75atm exhibit low pressure reactions, dominated by H, O, OH production and high pressure chemistry driven by HO2 and H2O2. Deflagration is observed for the lowest initial pressure. Increasing pressure results in smaller induction times and ignition, followed by a detonation wave. The spatial and temporal evolution of the gas bubble is highly affected by the type of ignition. The Richtmyer–Meshkov instability and the subsequent Kelvin–Helmholtz instabilities develop with a high reaction sensitivity. Mixing is significantly reduced by both reaction types. The strongest effect is observed for detonation.
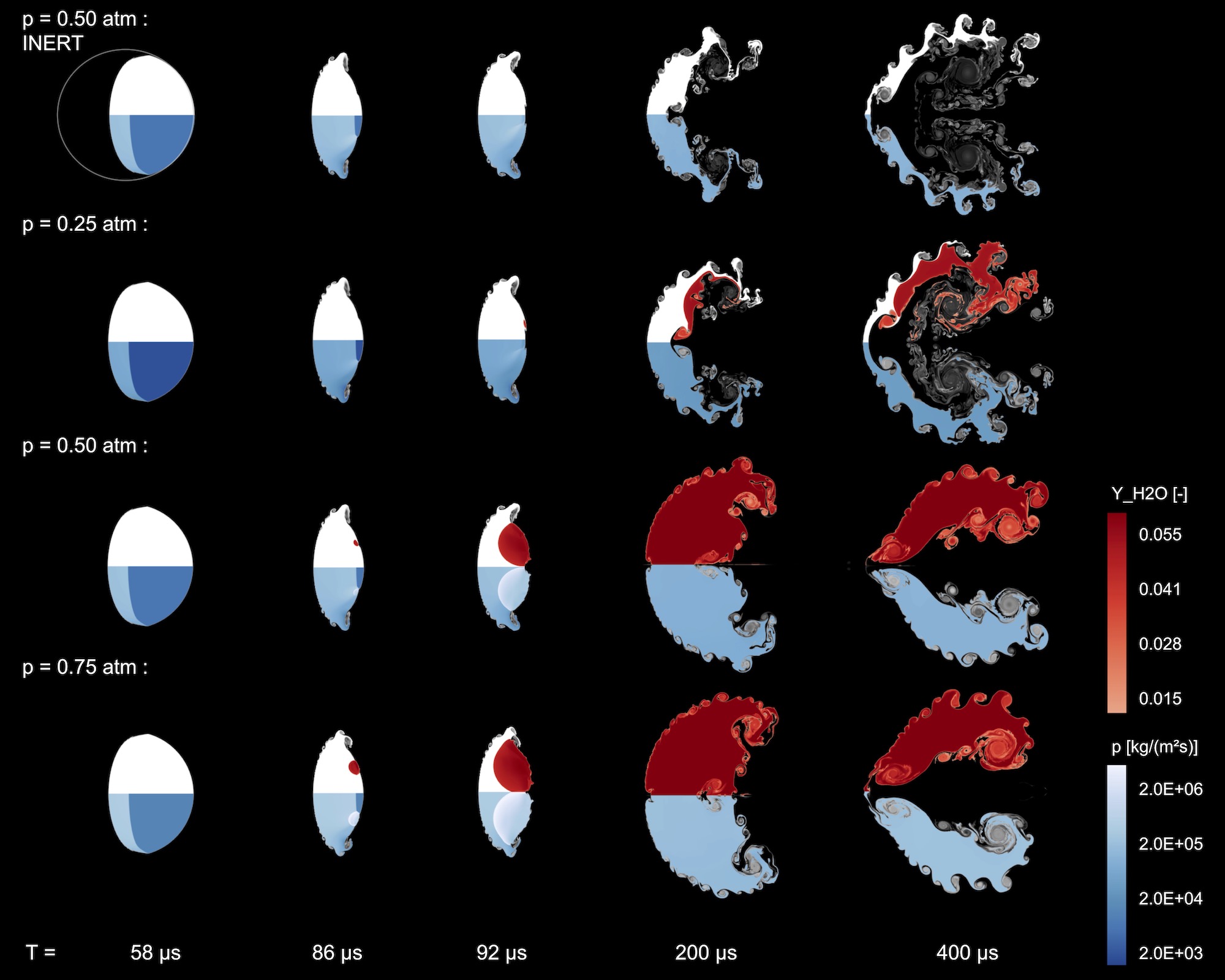
Chemical kinetics in reacting shock-bubble interactions at different atmospheric pressure levels: Upper part shows mass fraction of the inert gas Xe (gray scale) and the product H2O (red color scale), illustrating the reaction wave. Lower part depicts the characteristic pressure distribution.
